The ketogenic diet is the most popular dietary trend in our world today.
Especially for those living with diabetes, it’s likely that you’ve been tempted to follow a ketogenic diet to lose weight, drop your A1c, and flatline your blood glucose.
Even though it may seem tempting to enter the metabolic state of ketosis, it’s important to understand the caveats of ketosis, so that you fully understand your risks for developing long-term complications.
What is Ketosis?
So what exactly is a ketogenic diet? And why is ketosis a popular recommendation for those living with diabetes?
A ketogenic diet a very low-carbohydrate diet by design, containing a maximum of 30 grams of dietary carbohydrate per day.
When eating a ketogenic diet, you are told to avoid carbohydrate-rich foods like fruits, starchy vegetables, legumes, and whole grains, and instead eat larger quantities of meat, dairy, leafy greens, non-starchy vegetables, nuts, seeds, and vegetable oils.

At the base of the ketogenic food pyramid are eggs, dairy, meat, oil, and fish, which make up the bulk of calories eaten. Non-starchy vegetables contain too much carbohydrate energy and are avoided, while non-starchy vegetables or “green vegetables” are included, along with nuts, seeds, and very limited amounts of fruit (mainly berries).
In order to achieve the state of ketosis, you are only allowed to eat a small amount of carbohydrate energy from fruits and starchy vegetables.
The ketogenic diet explicitly prohibits the consumption of grain products (even whole grains), pasta, refined sugar, milk, corn, legumes (including lentils, beans, and peas), as well as rice.
When you eat a ketogenic diet, your muscle and liver switch from oxidizing glucose as their primary fuel to fatty acids as their primary fuel.
While this may seem like a “logical” approach to reducing blood glucose fluctuations, one significant problem with a ketogenic diet is that your brain cannot follow the lead of your liver and muscle by switching from glucose to fatty acids and amino acids.
Instead, your brain is designed to run off of glucose exclusively, and is therefore dependent on dietary carbohydrate for energy. In order to withstand a very low-carbohydrate intake, your liver manufactures ketone bodies as an emergency backup fuel for your brain when in the state of ketosis.
If you are living with diabetes, this may sound like a great idea, because your pancreas is provided with an opportunity to reduce insulin production due to low carbohydrate intake.
Millions of people around the world who eat a ketogenic diet, achieve a flatline blood glucose profile and greatly reduce or eliminate their need for oral medication and insulin.
If you have experienced this yourself, you may be thinking, “Great, I solved the problem. Eating a ketogenic diet is keeping my blood glucose in control, and therefore my diabetes health is going up.”
In addition, the state of ketosis induces a number of short-term benefits including rapid weight loss, reduced fasting glucose, reduced post-meal blood glucose, reduced A1c, reduced total cholesterol, reduced LDL cholesterol, and flatline blood glucose.
Medical professionals love seeing these biomarker changes, which tricks both patients and doctors into believing that ketosis is an excellent long-term dietary strategy.
The problem is that eating a ketogenic diet significantly increase your risk for chronic disease and premature death in the long-term, as you will read about in this article.
After researching the advice from the top ketosis gurus, we made a list of the 7 biggest (and most dangerous) misconceptions about ketogenic diets.
In this article, we’ll go into detail about the truth underlying ketosis, and refute many common statements backed by misleading science, incorrect biochemistry, and a fundamental lack of understanding of human biology.
Download the Free PDF: Debunking Ketosis and Ketogenic Diets

Click on the button below to download your free report – Ketosis and the Ketogenic Diet: Debunking 7 Misleading Statements
Ketosis Misconception #1: Insulin is Your “Fat Storage Hormone”
You may have heard people in the ketogenic community refer to insulin as your “fat storage hormone,” and that by adopting a very low-carbohydrate diet, you prevent your blood glucose from “spiking” after a meal.
While there is some truth to this statement, it is absolutely crucial to understand that the primary function of insulin is to help transport glucose out of your blood and into tissues, and a secondary effect of insulin is to help transport fatty acids and amino acids out of your blood and into tissues.
Simply because insulin has the ability to transport fat into tissues, does not mean that it’s factually correct to label insulin as your “fat storage hormone” – this is a gross exaggeration of the actual role of insulin, and is meant to scare people into believing that any amount of insulin in circulation will make you fat.
It is important to understand that insulin is most effective at promoting glucose uptake, but also functions to promote fatty acid uptake and amino acid uptake into tissues.
Open any biology textbook, and you’ll find that the primary function of insulin is to help glucose exit your blood and enter tissues, but that insulin also facilitates the movement of fatty acids and amino acids out of your blood and into tissues (1).
Labeling insulin as your “fat storage hormone” is simply incorrect from a biological perspective.
Insulin triggers macronutrient uptake in this order:
- Priority #1: Insulin transports glucose into tissues (a) to be immediately oxidized for energy or (b) to be stored as glycogen for later use
- Priority #2: Insulin transports fatty acids into tissues (a) to be immediately oxidized for energy or (b) to be stored as triglyceride for later use
- Priority #3: Insulin transports amino acids into tissues (a) to synthesize new protein or (b) to be burned for energy immediately
Take a look at this infographic for a more detailed understanding of the insulin priority hierarchy.
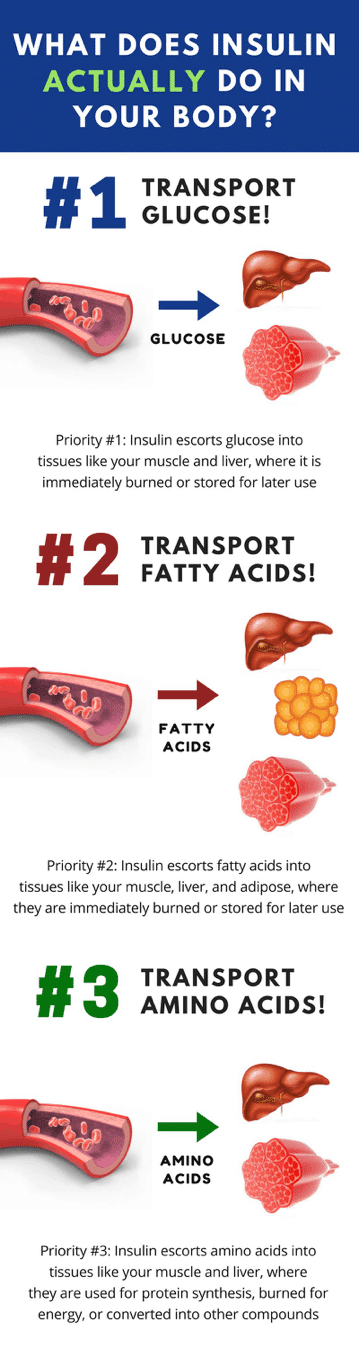
Understanding this insulin priority hierarchy is very important because it reinforces the concept that insulin’s primary role is to handle all things related to glucose metabolism before it begins directing fatty acids and amino acids into tissues.
Insulin is the most powerful anabolic hormone in your body (meaning that it promotes more growth and fuel storage than any other hormone), and ketogenic dieters exaggerate this fact, condemning insulin entirely, claiming that even small amounts of insulin will make you fat.
The truth is that all mammals secrete insulin, because insulin is absolutely required for life.
- Your dog secretes insulin.
- Your pet hamster secretes insulin.
- Your neighbor’s cat secretes insulin.
- Monkeys secrete insulin.
- Raccoons secrete insulin.
- Your non-diabetic coworker secretes insulin.
In fact, insulin is so important that if your body stops manufacturing it, you die. Without insulin, your dog would die. Your pet hamster would die. Your neighbor’s cat would die. Your non-diabetic coworker would die.
- Fact: insulin is the most anabolic hormone in your body, responsible for more fuel storage and cell growth than any hormone in your body.
- Fact: Insulin promotes more growth than testosterone.
- Fact: Insulin promotes more growth than estrogen.
- Fact: Insulin promotes more growth than growth hormone.
- Fact: Insulin promotes more growth than IGF-1.
In truth, a physiologically normal amount of insulin is required to stay alive, but secreting or injecting excess insulin is what substantially increases your risk for coronary artery disease, atherosclerosis, and cardiovascular disease as a whole (2–10).
Labeling insulin as your “fat storage hormone” not only misrepresents it’s true biological activity, it scares people into believing that insulin is the enemy, and even small amounts of insulin cause weight gain, obesity, and type 2 diabetes.
Ketosis Misconception #2: Eating Carbohydrates Spikes Your Blood Glucose
Proponents of the ketogenic diet often argue that eating any food containing carbohydrate energy will spike your blood glucose, and that the only way to avoid dangerous blood glucose spikes is to avoid carbohydrate-rich foods.
Technically speaking, when you eat carbohydrate-rich food, your blood glucose will rise. Furthermore, reducing your carbohydrate intake will keep your blood glucose more stable.
For these reasons, ketogenic dieters maintain a total carbohydrate intake of less than 30 grams per day, representing less than 10% of total calories on average.
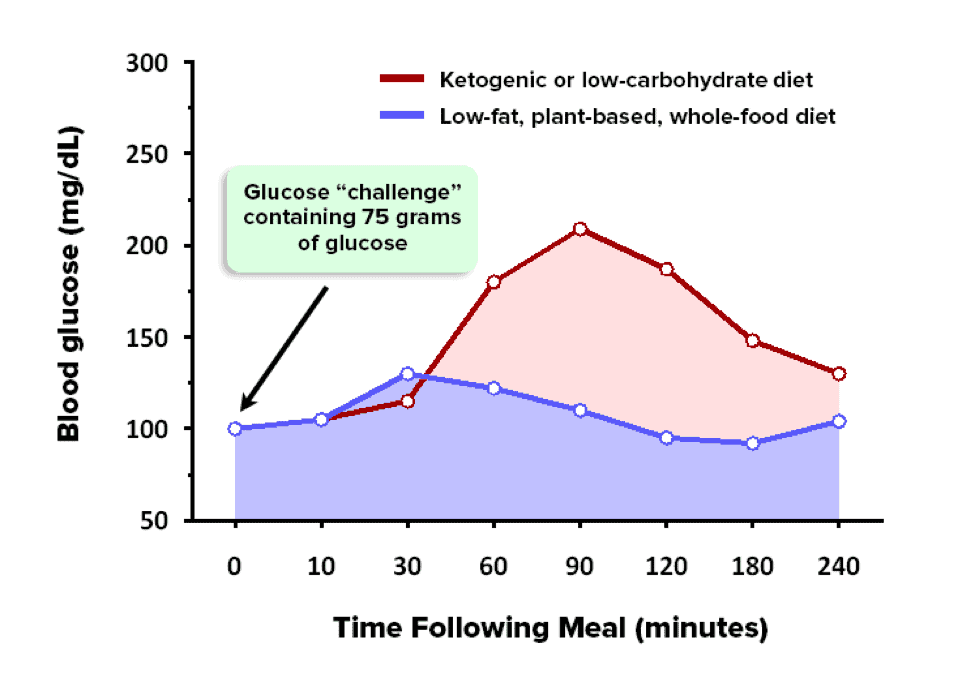
What those in ketosis don’t understand is that the amount of glucose in your blood is not only determined by the amount of carbohydrate that you eat, but instead is a reflection of both your dietary carbohydrate intake AND your dietary fat intake (11–30).
We have written extensively about the detrimental role that excess dietary fat plays in the development of insulin resistance, leading to high blood glucose, increased insulin requirements, high cholesterol, beta cell death, and increased risk for many chronic diseases.
Paying attention to your carbohydrate intake only will mislead you into thinking that this single macronutrient controls your blood glucose profile.
In reality, your blood glucose is determined primarily by how much fat you eat, and secondly by the amount of carbohydrate you eat.
To understand how your blood glucose responds to different macronutrient profiles, let’s explore how a ketogenic diet, Standard American Diet (SAD), and low-fat, plant-based, whole-food diet affect your blood glucose.
Diet #1: Ketogenic Diet
Suggested carbohydrate intake: <10% of calories (<30 grams per day)
Suggested fat intake: 60-70% of calories
Suggested protein intake: 20-35% of calories
When operating in a high-fat ecosystem on a ketogenic diet, the primary reason why your blood glucose remains flat is because of the near absence of carbohydrate-rich foods.
In this way, eating a high-fat diet is very effective at flatlining your blood glucose values because carbohydrates are kept below 30 grams per day. As long as you avoid carbohydrate-rich foods, your blood glucose will remain remarkably stable.
But the minute you chose to eat a carbohydrate-rich food (such as a banana, potato, or bowl of quinoa), your blood glucose is likely to increase significantly due to a hidden state of fatty-acid-induced insulin resistance.
For these reasons, those in ketosis often fail an oral glucose tolerance test, because their muscle and liver are incapable of metabolizing a carbohydrate challenge.
Diet #2: Standard American Diet (SAD)
Average carbohydrate intake: 40-45% of calories
Average fat intake: 40-45% of calories
Average protein intake: 15-20% of calories
The SAD is a perfect example of a diet that is high in both carbohydrates and fat, increasing your risk for high blood glucose, insulin resistance, and diabetes.
Because both fat and carbohydrate are present in large quantities, controlling your blood glucose becomes increasingly difficult over time.
Diet #3: Low-Fat, Plant-Based, Whole-Food Diet
Suggested carbohydrate intake: 70-80% of calories
Suggested fat intake: 10-15% of calories (20-30 grams per day)
Suggested protein intake: 10-15% of calories
Because a low-fat, plant-based, whole-food diet is low in dietary fat, your carbohydrate tolerance (ability to eat carbohydrate-rich food) increases substantially, resulting in maximal insulin sensitivity, and the opportunity to completely reverse insulin resistance.
When operating in a low-fat ecosystem on a plant-based diet, it is quite easy to maintain flatline blood glucose as long as your total fat intake is maintained below 30 grams per day, and your carbohydrate intake comes from whole foods like fruits, vegetables, legumes, and whole grains and not from products containing refined sugars.
Ketosis Misconception #3: Diabetes is Carbohydrate Toxicity, and Insulin Resistance is a State of Carbohydrate Intolerance
Those in the ketogenic community often label diabetes as a problem of “carbohydrate toxicity,” suggesting that dietary carbohydrate is the primary cause of the disease process.
In addition, ketogenic dieters believe that insulin resistance is caused by insulin itself, triggered by an excess consumption of dietary carbohydrate.
In order to make these statements factually correct, it’s necessary to go back to basic biochemistry principles and understand that the vast majority of people who develop insulin resistance do so by eating a diet containing large amounts of dietary fat.
The research world has known for more than 85 years that carbohydrate intolerance results from the overconsumption of fat. This was first established in the 1930’s by the pioneering work of Drs. Rabinowitch (31–34) and Himsworth (35), then further proven by Dr. Kempner (36) in the 1950’s, and by Dr. Anderson (37–39) in the 1970’s.
You can read all about those studies by clicking on the image below:
Despite this, the cause of insulin resistance and carbohydrate intolerance remains one of the most debated subjects in the world of diabetes even today.
The problem is that by labeling carbohydrate intolerance as a problem of carbohydrate toxicity rather than a problem of fat toxicity, most people are driven towards a low-carb diet to stop them from needing more oral medication and insulin over time.
Even though a high-fat, high-protein diet is very effective at reducing blood glucose fluctuations in the short term, many people become more insulin resistant over the course of years. In this respect, the short-term benefits do not accurately reflect the long-term consequences of eating a low-carbohydrate diet.
Think of insulin resistance as a series of metabolic dominoes. The dominoes are arranged in this order:
1. Eat excess dietary fat (greater than 15% of total calories)2. Dysfunctional insulin receptors
3. Glucose becomes “trapped” in your blood, resulting in high blood glucose
4. Your need for oral medication and/or insulin increases
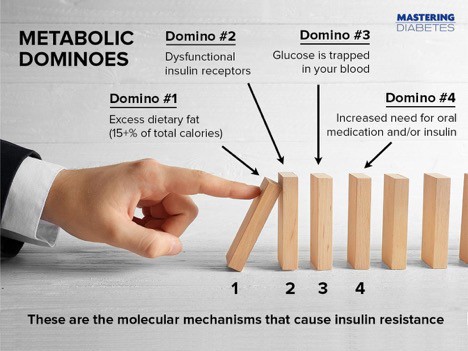
The problem is simple: if you talk about carbohydrate intolerance without talking about fat, you’re missing the bigger picture.
The correct statement is this: Insulin resistance is a state of carbohydrate intolerance, created by the consumption of excess dietary fat.
As I demonstrated in a recent lecture, how this works is that the consumption of excess fat blocks the ability of insulin receptors in your muscle and liver to allow glucose to exit the blood and enter these tissues.
Glucose then gets trapped in the blood, resulting in high blood glucose and an increased need for insulin.
Ketosis Misconception #4: Carbohydrate is Not an “Essential Nutrient”
The ketogenic world is quick to point out that there is no such thing as an "essential carbohydrate,” in contrast to required nutrients like essential fatty acids and essential amino acids.
While this statement is technically true, labelling glucose as a "nonessential" carbohydrate implies that there is no use for glucose in the human body.
Once again, let’s return to basic human physiology in order to understand the truth.
Your muscle, liver, and other peripheral tissues are capable of oxidizing glucose, amino acids, and fatty acids for energy. Your brain, however cannot oxidize either amino acids or fatty acids for energy.
Your brain can only burn glucose for energy, and does not possess the biological machinery to store glucose.
As a result, your brain must oxidize glucose on-demand, importing glucose from your blood 24 hours a day. Since glucose is your brain’s principal on-demand fuel, carbohydrate-rich foods are your brain’s primary fuel source.
When you consume a low-carb diet, you force your liver to synthesize an emergency backup fuel known as ketone bodies to prevent against brain starvation.
In this state, your brain is forced to adapt to a new emergency backup fuel, and you enter the state of ketosis in which ketone bodies are your brain’s principal fuel.
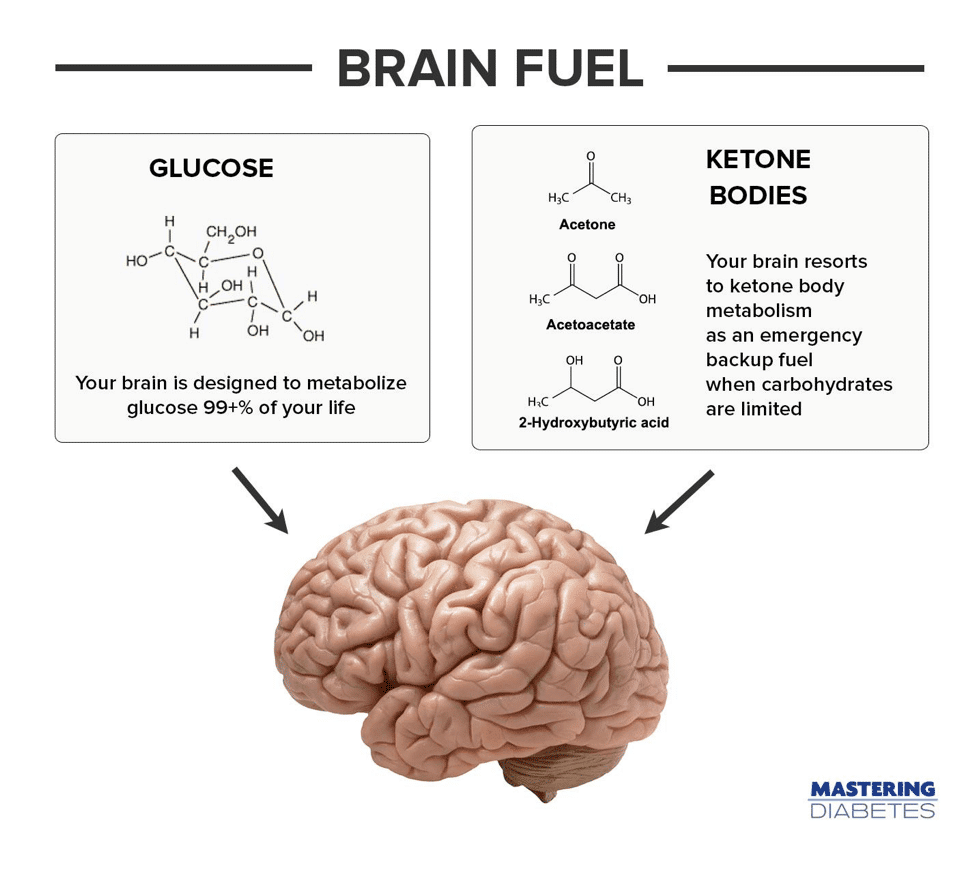
Ketogenic diets were originally invented for people with epilepsy, and are effective at reducing seizure incidence. However, ample evidence shows that ketogenic diets come with a laundry list of unwanted side effects that simply cannot be overlooked (40,41).
These side effects are chronic health conditions that are fueled by a diet low in carbohydrates, high in fat, high in protein, and low in micronutrients like vitamins, minerals, and antioxidants.
The list of ketosis side effects is here:
Side Effects of Ketogenic Diets
- Diarrhea
- Nausea
- Constipation
- Vomiting
- Acid reflux
- Hair loss
- Kidney stones
- Muscle cramps or weakness
- Hypoglycemia
- Low platelet count
- Impaired cognition
- Inability to concentrate
- Impaired mood
- Renal tubular acidosis
- Disordered mineral metabolism
- Stunted growth in children
- Increased risk for bone fractures
- Osteopenia and Osteoporosis
- Increased bruising
- Sepsis
- Pneumonia
- Acute pancreatitis
- Hyperlipidemia
- High cholesterol
- Insulin resistance
- Elevated cortisol
- Increased risk for cardiovascular disease
- Increased risk for atherosclerosis
- Cardiomyopathy
- Heart arrythmia
- Myocardial infarction
- Menstrual irregularities
- Amenorrhea (loss of period)
- Increased risk for all-cause mortality
Most importantly, ample scientific evidence clearly shows that low-carbohydrate diets increase your risk for all-cause mortality, or premature death from any cause (42–45,45–50).
Yes, that’s right – eating a low-carbohydrate diet can shorten your lifespan, and that’s very important to understand.
It is important to understand that the statement that carbohydrates are “nonessential” is not only factually inaccurate, it results in adopting a low-carbohydrate diet or ketogenic diet that increases your risk for a wide variety of chronic health conditions that may ultimately shorten lifespan, decrease your quality of life, and accelerate your risk for chronic disease.
Ketosis Misconception #5: Low Fasting Insulin Means High Insulin Sensitivity
People in the ketogenic community often measure their fasting insulin levels as an indicator of their glucose metabolism. A fasting insulin test measures the amount of insulin your pancreas must secrete in order to control your blood glucose.
The lower the number, the less work your pancreas is performing. This is a good thing.
Ketogenic dieters often report very low fasting insulin levels, then draw the conclusion that their “insulin sensitivity has increased.” This could not be farther from the truth.
The only way to actually measure your insulin sensitivity is to utilize a glucose “challenge” in which you either drink a solution containing glucose dissolved in water or eat a food containing carbohydrate energy.
In the clinic, your doctor may order a glucose tolerance test to measure your insulin sensitivity. The way that you measure insulin sensitivity using an oral glucose tolerance test is straightforward:
- Step 1: A patient drinks a solution containing 75 or 100 grams of glucose dissolved in water.
- Step 2: A medical professional samples blood at 0, 60, 120 and 180 minutes
- Step 3: Blood samples are analyzed for glucose and insulin concentrations
- Step 4: Your performance is measured against a standard to determine your insulin sensitivity
The higher your glucose and insulin area under the curve (AUC), the worse you perform on the test, and the higher your level of insulin resistance. The lower the glucose and insulin AUCs, the lower your level of insulin resistance.
The reason why this test is so valuable for measuring insulin sensitivity is because it measures the ability of your muscle and liver to uptake glucose from your blood when challenged by a food or drink containing glucose.
Simply measuring your fasting insulin or fasting blood glucose level (independent of a glucose “challenge”) is insufficient information to conclude anything about your level of insulin sensitivity, however many ketogenic dieters and medical professionals fail to understand this concept entirely.
If you never challenge your glucose metabolism with carbohydrate-rich foods or with a glucose solution, it is impossible to measure insulin resistance.
Despite this, those in ketosis often claim that their insulin sensitivity has increased even though they avoid eating carbohydrate-rich foods at all costs.
In addition, those eating a ketogenic diet fail to understand that eating a low-carbohydrate diet and living in a state of ketosis itself increases your likelihood of failing a glucose tolerance test significantly.
Unfortunately, those in the low-carbohydrate world don’t seem to understand this concept, and as a result they often avoid a glucose tolerance test altogether, continually mistaking lower fasting insulin for improved insulin sensitivity.
Ketosis Misconception #6: Low-Carbohydrate Diets are Not High-Protein Diets
Another misleading statement in the world of ketogenic diets is that “a ketogenic diet is not a high-protein diet.” Let’s go into detail to understand the caveats of this statement.
It is important to understand that the term “high-protein” is widely debated. Plant-based advocates argue that anything greater than 15% of total calories increases your risk for chronic disease, whereas animal-based advocates claim that a protein intake as high as 25% does not increase your risk for chronic disease in the long-term.
The first question to ask is this: what proportion of total calories constitutes a high-protein diet according to the scientific evidence?
According to the evidence-based research, diets containing more than 10-15% of total calories in protein increase your risk for cardiovascular and diabetes mortality, especially if the majority of your protein originates from animal foods (45).
The Standard American Diet (SAD) contains on average 16% calories from protein, and many people eat between 20-25% of their diet in protein. Many studies have shown that protein intakes in excess of 15% of total calories increase your risk for heart disease, high cholesterol, atherosclerosis, diabetes, and various forms of cancer.
We recently wrote an article showing the results of a study in which a high-protein diet impaired insulin sensitivity in patients undergoing weight loss, which shows that increasing protein intake adversely effects insulin resistance.
As a result, any diet containing an excess of 10-15% calories from protein is considered a high-protein diet.
As we discussed earlier, in order to maintain the state of ketosis, your diet must contain less than 30 grams of carbohydrate per day, and contain an average of 60-70% calories from dietary fat and 20-35% calories from protein. This, by definition is a high-protein diet.
It turns out that it is practically impossible for a ketogenic diet to be low in protein. Why? It’s actually quite simple.
Cheese, eggs, meat, butter, poultry, fish, nuts, seeds, vegetable oil, coconuts and avocados make up the bulk of calories in the ketogenic diet. With the exception of vegetable or coconut oil (100% fat), every food listed above is not only high in fat, but also high in protein.
Any attempt at constructing a low-carbohydrate diet using these foods as your principal energy source will result in a protein intake greater than 10-15%, unless the bulk of your calories are derived mainly from vegetable or coconut oil.
Ketosis Misconception #7: Evidence-Based Research Shows that Low-Carbohydrate Diets are Effective
Low-carbohydrate advocates are masters of documenting the efficacy of their philosophy using studies with small population sizes conducted over short time periods – often over weeks or months.
While these studies are helpful at assessing the short-term benefits of ketosis, they fail to document the long-term effects of a ketogenic diet.
A classic example of this is a paper published in 2017 documenting the results of 10 weeks of a ketogenic diet on 262 patients following a diet containing less than 30 grams of carbohydrate per day, and an average of 175 grams of protein per day (51).

These researchers document how 10 weeks of ketosis resulted in an average A1c decrease of 1.0%, an average weight loss of 7.2%, and how more than 56% of participants reduced their need for oral medication.
These are all great outcomes. The problem is that the study was conducted in a small cohort over a very short time period.
In order to determine the true effectiveness of any diet, you have to do two things:
- 1) Study your diet in large numbers of people (tens or hundreds of thousands of people)
- 2) Study the outcomes of people following your diet over long periods of time (5+ years)
Studies conducted in tens of thousands of people over 5+ years indicate that low-carbohydrate diets have the following disastrous outcomes (42–45,45–50):
- Increased cardiovascular disease
- Increased risk for hemorrhagic stroke
- Increased risk for atherosclerosis
- Increased risk for diabetes mortality
- Increased risk for obesity
- Increased risk for all-cause mortality (premature death)
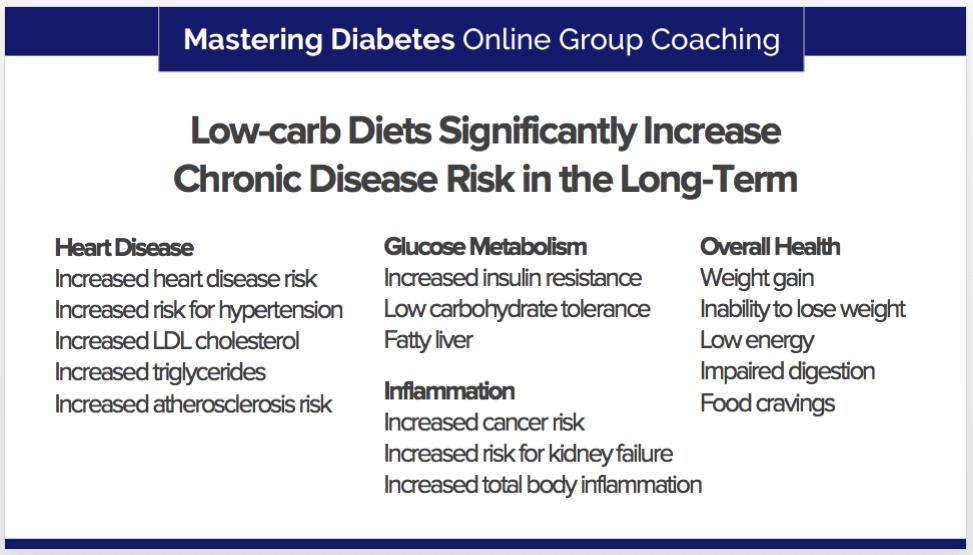
No matter how you slice it, low-carbohydrate diets trick patients and doctors into believing that ketosis is an excellent long-term dietary strategy, when in reality the long-term consequences are often worse than the initial condition they were designed to reverse.
We recently published an article documenting the grim long-term effects of low-carbohydrate diets, in which we explain the evidence-based research showing that low-carbohydrate diets high in fat and protein including meat, dairy products, eggs, fish, and oil actually worsen diabetes health, increase cancer risk, increase cholesterol, increase atherosclerosis, harden blood vessels, and increase all-cause mortality.
Take Home Messages
The next time someone asks you, “What’s your take on ketosis?” now you have the knowledge to speak from an evidence-based perspective.
Here’s a summary of what I’ve covered. Each misleading statement is followed by the evidence-based correction:
Ketosis Misconception #1: Insulin is your fat storage hormone.
Mastering Diabetes: The primary function of insulin is to help transport glucose out of your blood and into tissues, and a secondary effect of insulin is to help transport fatty acids and amino acids out of your blood and into tissues.
Ketosis Misconception #2: Eating carbohydrates spikes your blood glucose.
Mastering Diabetes: As long as you avoid carbohydrate-rich foods, your blood glucose will remain remarkably stable. But the minute you chose to eat a carbohydrate-rich food (such as a banana, potato, or bowl of quinoa), your blood glucose is likely to increase significantly due to a hidden state of fatty-acid-induced insulin resistance.
Ketosis Misconception #3: Diabetes is a problem of carbohydrate toxicity, and insulin resistance is a state of carbohydrate intolerance.
Mastering Diabetes: Insulin resistance is a state of carbohydrate intolerance, created by the consumption of excess dietary fat.
Ketosis Misconception #4: Carbohydrate is not an essential nutrient.
Mastering Diabetes: This statement is not only factually inaccurate, it results in adopting a low-carbohydrate diet or ketogenic diet that increases your risk for a wide variety of chronic health conditions that may ultimately shorten lifespan, decrease your quality of life, and accelerate your risk for chronic disease.
Ketosis Misconception #5: Low fasting insulin means high insulin sensitivity.
Mastering Diabetes: In the absence of performing a glucose tolerance test to quantify a patient’s insulin sensitivity, you cannot conclusively determine whether you are insulin resistant or insulin sensitive.
Ketosis Misconception #6: Low-carbohydrate diets are not high-protein diets.
Mastering Diabetes: Protein intakes in excess of 10-15% of total calories increase your risk for heart disease, high cholesterol, atherosclerosis, diabetes, and various forms of cancer. It is nearly impossible to eat a low-carb diet that doesn’t contain more than 15% calories from protein.
Ketosis Misconception #7: Evidence-based research shows that low-carbohydrate diets are effective
Mastering Diabetes: Studies conducted in tens of thousands of people over 5+ years indicate that low-carbohydrate diets increase your risk for cardiovascular disease, hemorrhagic stroke, hypertension, atherosclerosis, diabetes mortality, obesity, cancer, and all-cause mortality (premature death). No matter how you slice it, low-carbohydrate diets trick patients and doctors into believing that ketosis is an excellent long-term dietary strategy, when in reality the consequences can be disastrous.
Lower Your A1c and Get to Your Ideal Body Weight ... Guaranteed

Your results are guaranteed. Join more than 10,000 ecstatic members today
Personalized coaching puts you in immediate control of your diabetes health, helps you gain energy, improves your quality of life, and reduces or eliminates your meds.
References
Leave a Comment!
*Please note: We welcome the discussion of varying and conflicting viewpoints. However, we will not tolerate name calling, cursing, or other words of "attack." Thank you for your understanding.


