
An overwhelming amount of scientific evidence shows that a high-fat diet is the single most effective method at killing beta cells and inducing insulin resistance in both your liver and muscle.
The evidence clearly demonstrates that increasing dietary fat intake has an immediate negative effect on insulin sensitivity, which can then develop into a chronic state of insulin resistance and eventually diabetes if the quantity of dietary fat remains high (1–26). For more information on this topic, watch this video.
Many people ask me about the specific mechanism that links dietary fat to beta cell death. Let’s explore this in detail.
What are Beta Cells and Why are They Important?
If insulin were not present, glucose would accumulate in your blood, and aggravate tissues all throughout your body by increasing inflammation in all tissues.
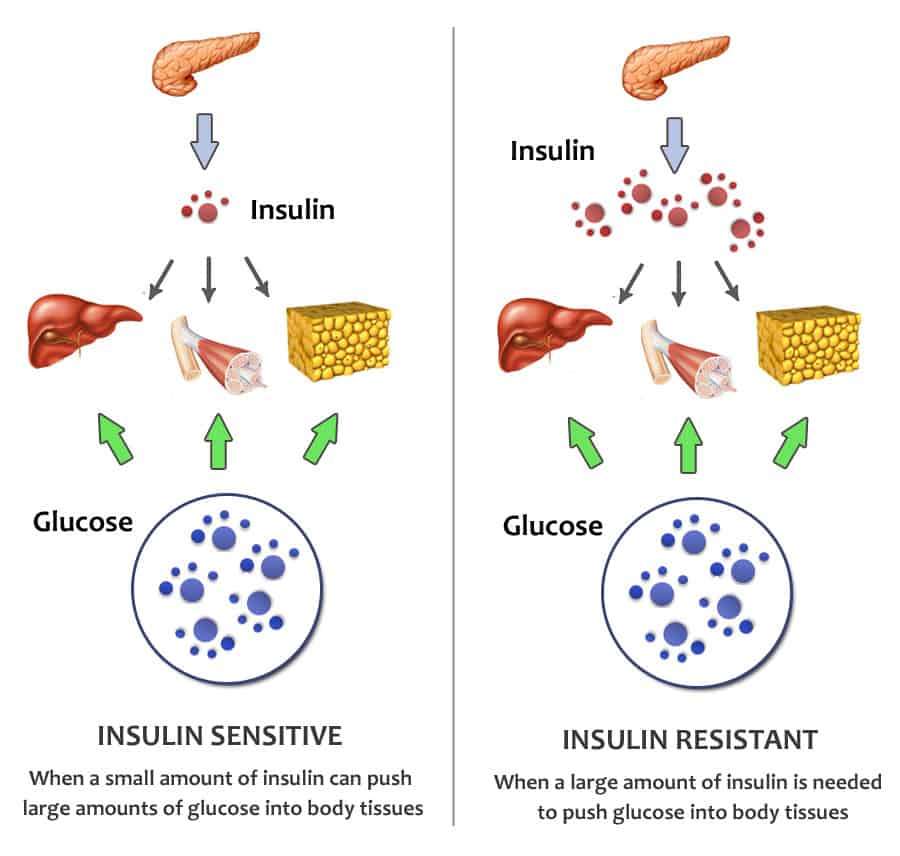
Think of insulin as a required glucose escort, whose job is to usher glucose out of your blood and into tissues.
Without insulin, tissues would be starved for glucose.
Because insulin is so crucial, jeopardizing insulin production results in severe metabolic problems that can then lead to whole-body organ dysfunction and eventually death.
Because of this, protecting beta cell health throughout life is critical for your long-term health.
What Causes Beta Cell Dysfunction?
It turns out that beta cells in your pancreas are highly susceptible to fat accumulation, similar to your liver and muscle.
Known as lipotoxicity, the accumulation of excess fat in your beta cells leads to severe beta cell dysfunction and cell suicide (27–38).
In comparison with cells in your liver and muscle, beta cells are particularly sensitive to dietary fatty acids because they have a limited ability to protect themselves against fatty acid accumulation.
When exposed to high fat concentrations for long periods of time, their antioxidant self-defense mechanisms are inadequate to protect them against dysfunction (33,34).
Step 1: Stressed Beta Cells Make Excess Insulin
The way that beta cells behave depends on a number of factors, including fat concentration, glucose concentration, and the amount of time that they are exposed to high levels of either fat or glucose (34).
As fat spills over from adipose tissue and the level of whole-body insulin resistance increases over time, your pancreas responds by making more insulin to overpower your muscle and liver into behaving properly. In effect, your beta cells are saying…
“Wow the amount of glucose in the blood is incredibly high. I better make more insulin so that the liver and muscle will have no choice but to take it up. When the going gets tough, the tough make insulin!”
Because the beta cells are now overproducing insulin beyond their physiologically normal level, they enter a state of cellular stress.
As this cycle continues and the degree of insulin resistance increases over time, the amount of insulin produced by your pancreas also continues to increase.
Step 2: Beta Cells Maximize Insulin Production
At a certain point, insulin production no longer increases – your beta cells are sufficiently stressed and their production of insulin is maximized.
At this point, your beta cells simply cannot make more insulin. In some individuals, this process can take many years to develop, and in others this process occurs very quickly.
The amount of insulin produced at the peak is highly variable between individuals; some people hit peak insulin production at 150% of normal whereas others hit peak insulin production at 450% of normal.
The amount of insulin produced at peak depends on both the number of beta cells and the strength of the beta cells, both of which are variable between individuals.
More insulin is capable of being produced as both the size of the beta cell population and the relative strength each individual beta cell increases.
Despite these individual differences, the common thread between all insulin resistant individuals is that beta cells stress triggers excess insulin production beyond their physiological normal amount.
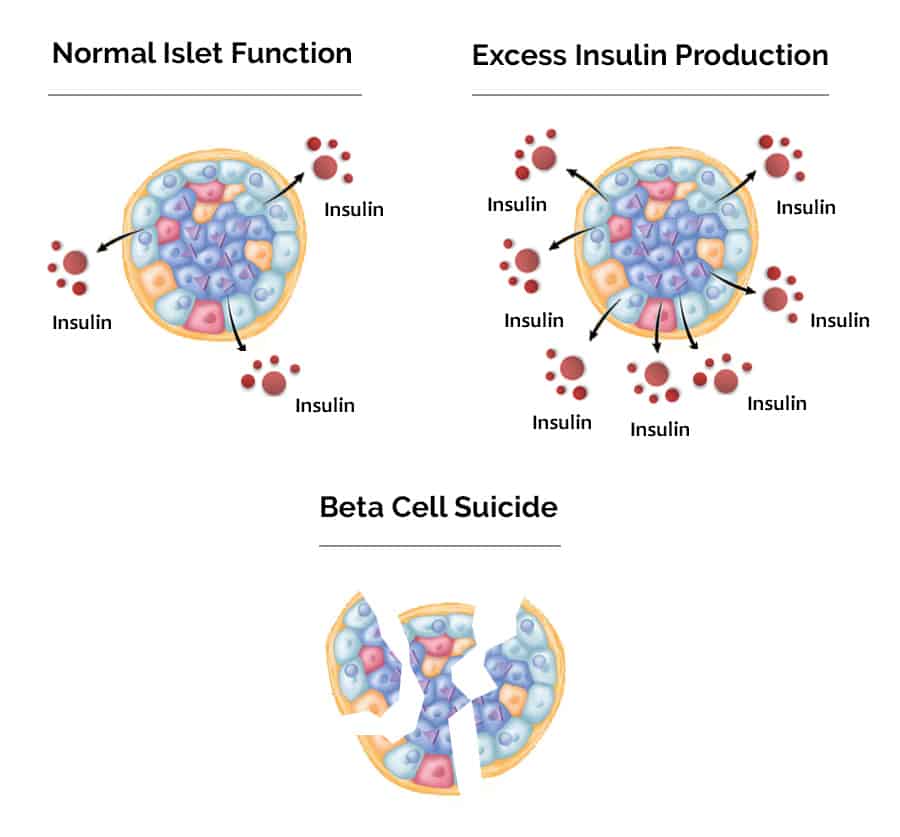
Step 3: Stressed Beta Cells Commit Suicide
There comes a point in the life of a stressed beta cell where it is more advantageous to commit suicide than it is to stay alive.
At this point, your beta cells simply cannot make more insulin. In some individuals, this process can take many years to develop, and in others this process occurs very quickly.
When a large population of stressed beta cells commit suicide together, insulin production falls rapidly in a short period of time.
As a result of this massive die off, insulin production falls to below normal physiological levels.
This state is called type 2 diabetes.
Some individuals retain 60% of their original beta cell mass whereas others will drop to as low as 20% of their original beta cell mass.
Autopsies have revealed that in the majority of patients with type 2 diabetes, more than half of the beta cell population has been permanently killed off (22).
In this state, only a small population of beta cells are now responsible for secreting enough insulin to satisfy whole-body insulin requirements.
As you may be able to predict, this job is extremely difficult unless you help your muscle and liver significantly reduce their requirement for insulin. Fortunately, these remaining beta cells are strong enough to remain alive, however they are at risk for death as long as insulin resistance persists.
Up until recently, researchers believed that after the age of twenty, your body stops making new beta cells (39). New research has revealed that making new beta cells is possible following the fasting mimicking diet (40).
The question then becomes this: if the level of insulin resistance is significantly reduced, can the remaining beta cell population produce enough insulin to meet the demands of your entire body?
In other words, are the remaining “beta cell soldiers” strong enough to withstand the test of time?
Fortunately for you, the answer is almost always yes. Even when beta cell mass has been significantly compromised, the remaining beta cell population is often capable of producing sufficient insulin for all tissues.
But in order to do this, the most effective approach is to reduce your level of liver and muscle insulin resistance by reducing your intake of dietary fat, otherwise the remaining beta cells remain stressed and will continue to commit suicide.
Below is a picture to summarize the process of beta cell death:
Step 4: Reverse Insulin Resistance Before It's Too Late!
A low-fat, plant-based, whole food lifestyle is the most powerful method of reducing whole-body insulin resistance and preserving long-term beta cell function. Period. End of story.
People that focus on reversing insulin resistance and maximizing insulin sensitivity benefit from a reduced biological need for insulin, to preserve the function of the remaining beta cell population.
Those living with type 1 diabetes (like Lindsay, Jessica, and Chris) are able to reduce their use of insulin while increasing their whole carbohydrate intake.
Those living with prediabetes and type 2 diabetes (like Bob, Tami, and Don) are able to reduce their use of oral medications, lose weight, and reverse type 2 diabetes completely.
And those that incorporate amla into their diet on a daily basis can reduce their blood lipids dramatically in a short period of time.
Adopting a low-fat, plant-based, whole-food lifestyle can maximize your insulin sensitivity and significantly improve your blood glucose control, while reducing your overall chronic disease risk.
Lower Your A1c and Get to Your Ideal Body Weight ... Guaranteed

Your results are guaranteed. Join more than 10,000 ecstatic members today
Personalized coaching puts you in immediate control of your diabetes health, helps you gain energy, improves your quality of life, and reduces or eliminates your meds.

