Ozempic, a medication used to improve blood sugar control in type 2 diabetes, has gained tremendous popularity in recent years due to its off-label use for weight loss.
However, it's essential to understand the potential dangers associated with this drug, including its numerous side effects and the black box warning issued by the FDA.
This article will delve into the biological mechanism of Ozempic, its popularity, and the reasons behind its concerning side effects.
Understanding the Biological Mechanism of Ozempic
Ozempic, also known as semaglutide, is a glucagon-like peptide-1 (GLP-1) receptor agonist. It works by mimicking the functions of a natural hormone in our body called GLP-1, which regulates blood sugar levels, insulin production, and appetite.
This class of medications function by mimicking the hormone GLP-1, a peptide hormone secreted by the L cells in the small intestine. In response to food intake, GLP-1 crosses the blood brain barrier and signals directly to the hypothalamus to reduce hunger signals to slow the ingestion of calories.
In addition, GLP-1 activates stretch receptors in the small intestine, which in turn signal to the hypothalamus to reduce appetite and increase energy expenditure. Additionally, GLP-1 helps reduce blood glucose concentrations by stimulating pancreatic insulin secretion.
Why are Ozempic and Other GLP-1 Agonists So Popular?
GLP-1 receptor agonists (GLP1-RAs) have gained popularity due to their effectiveness in managing type 2 diabetes and promoting weight loss.
They go by the names of Ozempic, Mounjaro, and Trulicity – those are the best-selling GLP-1 drugs, accounting for 70% of sales in 2023. Other popular GLP-1 drugs include:
Originally developed to reduce blood glucose in type 2 diabetes only, research published in the New England Journal of Medicine in 2021 documenting the powerful weight loss effects of the active ingredient semaglutide began the movement to prescribe GLP-1 receptor agonists as an off-label weight-loss drug.
The Science Behind the Popularity
In 2021, researchers from Northwestern University published the landmark STEP-1 trial in the New England journal of Medicine (1). The study investigated the effect of once-weekly 2.4mg semaglutide injection in 1961 non-diabetic patients with either (a) a BMI of 30 or higher, or (b) a BMI of 27 along with weight-related comorbidities.
Researchers discovered that after 68-weeks, those who received semaglutide experienced an average weight loss of 14.9%, while those who received a placebo injection experienced a weight change of only 2.4%. In total, 86.4% of the semaglutide group lost a minimum of 5% of their body weight, with minimal adverse effects.
The STEP 2 trial, published in The Lancet, investigated the effect of a standard 1.0 mg dose of semaglutide vs. a higher 2.4 mg dose in comparison with matched placebos over 68 weeks in 1210 participants (2). A 2.4 mg weekly injection resulted in 9.64% weight loss, a 1.0 mg weekly injection resulted in 6.99% weight loss, and a placebo injection resulted in 3.42% weight loss. The higher dose also achieved slightly better glycemic control, reductions in cardiometabolic risk, and improved physical function relative to the standard dose.
In the STEP 3 trial, 611 participants were randomly assigned to receive either semaglutide 2.4 mg or placebo in addition to intensive behavioral therapy to support a healthier lifestyle (3). Their results demonstrated that those who received semaglutide experienced an average weight reduction of 16.0% after 68 weeks, compared to 5.7% for those receiving placebo. Additionally, 86.6% of those treated with semaglutide achieved a 5% reduction in bodyweight, compared with 47.6% of those receiving placebo.
In the STEP-4 trial, 902 participants received semaglutide 2.4 mg for the initial 20 weeks, and were then randomly assigned to receive either (a) semaglutide or (b) a placebo for the remaining 48 weeks (4). Those who continued taking semaglutide lost an additional 7.9% body weight, resulting in a total weight loss of 17.4% over the entire trial. Those who switched to the placebo regained an average of 6.9% of their initial weight, resulting in a total weight loss of 5.0%.
The STEP-5 trial, published in Nature Medicine, evaluated the long-term efficacy of semaglutide 2.4 mg versus placebo in maintaining weight loss over a 2-year period in 304 participants (5). The study found that semaglutide resulted in significant weight loss until week 60, with the effects maintained through week 104. At the end of the 2-year period, there was an average placebo-corrected weight loss of 12.6%.
These investigations provided strong evidence that semaglutide is an effective therapy for both weight loss and type 2 diabetes, and the combined benefits have led to a surge in prescriptions, reaching approximately 40 million in the U.S. in 2022.
The Dangerous Side Effects of Ozempic
While weight loss can bring about significant health benefits, it's important to note that rapid weight loss can have negative consequences, including a decrease in muscle mass, lower bone density, and a decrease in resting metabolic rate (RMR). The combined effects can increase the risk for sarcopenia – the gradual loss of muscle mass, strength, and function.
Physiologists have discovered that when people lose weight, about 25-30% of their weight loss is lean muscle mass, and the remainder is a reduction in fat mass. In the STEP-1 study, patients treated with semaglutide lost nearly 14 kg in total, with 8.5 kg (60%) being fat loss and 5 kg (38%) being lean mass loss, which exceeds normal physiological expectations.
An analysis of 18 randomized control trials comparing the effects of GLP-1 RAs, SGLT-2 inhibitors, and Metformin demonstrated that semaglutide, dapagliflozin, ipragliflozin, and canagliflozin showed a significant weight loss in comparison with placebo treated controls (6).
In addition to lean body mass loss, other gastrointestinal side effects have also been observed including constipation, diarrhea, nausea, vomiting, and abdominal pain, feeling excessively full, excessive bloating and belching, and heartburn.
Researchers from British Columbia analyzed a random sample of 16 million patients from the PharMetrics Plus for Academics database (IQVIA), a large health claims database that captures 93% of all outpatient prescriptions and physician diagnoses in the US between the years 2006-2020. They included patients with a recent history of obesity, and excluded those with diabetes or who had been prescribed another antidiabetic drug.
They found that the administration of GLP-1 agonists is associated with:
These results are similar to those of a study investigating the long-acting GLP-1 RA liraglutide. A 16-week randomized study demonstrated that liraglutide significantly delayed the gastric emptying of solids over a 16 week period (7).
Ozempic Has a Black Box Warning
The FDA issued a boxed warning (previously called a Black Box Warning) for Ozempic due to its potential to cause thyroid tumors and thyroid cancer. A boxed warning is the most serious safety warning issued by the FDA and not something to take lightly.
The black box warning was given to Ozempic because of the increased risk of thyroid C-cell tumors, including medullary thyroid carcinoma. This risk is higher in patients with a history of multiple endocrine neoplasia syndrome type 2 or patients with a family history of medullary thyroid carcinoma.
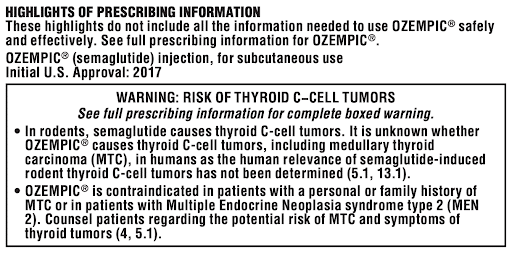
The warning label inside the packaging warns of the following risks:
Given the risks, it’s important to be very careful when choosing to use this to lose weight or lower your blood glucose, given the effectiveness of changing your diet and lifestyle.
How to Lose Weight and Keep it Off Permanently
Research has demonstrated that the most effective way to lose weight and keep it off in the long-term is via lifestyle modification, including a low-fat, low-energy diet containing between 1,300-1,700 kcal per day (8).
Interestingly, another study identified that in a population that lost an average of 30 kg for 5.5 years, only 4.3% reported using medication as part of their weight loss strategy (9).
A systematic review published in 2019 demonstrated that the most frequently reported habits of those successful at long-term weight loss and maintenance included having healthy foods available at home, regular breakfast intake, increasing vegetable consumption, decreasing sugary and fatty foods, limiting certain foods, and reducing fat in meals.
They also found that increased physical activity was the most accurate predictor of weight loss maintenance (10).
Take Home Messages
While GLP-1 RAs have shown promise in improving glycemic control and weight loss in individuals with type 2 diabetes, lifestyle modification is likely to be a more effective long-term solution. We wrote about that in a previous blog article, called The Low-Fat Diet for Diabetes.
The benefits of lifestyle modification, such as improved insulin sensitivity, weight loss, and reduced risk of cardiovascular disease, are not limited to the short-term and can have a lasting impact on overall health.
Additionally, lifestyle modification can be more cost-effective and has fewer side effects compared to GLP-1 RAs.
Ozempic may be a useful tool in the short-term, but it should always be used in conjunction with lifestyle modification to achieve optimal results.
To learn more about Ozempic and the dangers associated with its use, read this article and watch this video.
Lower Your A1c and Get to Your Ideal Body Weight ... Guaranteed

Your results are guaranteed. Join more than 10,000 ecstatic members today
Personalized coaching puts you in immediate control of your diabetes health, helps you gain energy, improves your quality of life, and reduces or eliminates your meds.

